|
|
PPE Information TCEP providing free N95 masks to members - deadline to sign up is October 21,2020 #GetUsPPE Project N95 Regional Advisory Councils - RAC ACEP Policy Statement on COVID19 PPE |
Statewide Updates TCEP/ACEP Letter to HHS regarding PPE and Physician Pay April 6, 2020 ACEP Strongly Supports Emergency Physicians who Advocate for Safer Working Conditions amidst Pandemic March 30, 2020 State Response to COVID-19 |
Federal Updates ACEP - Provider Relief Fund Update ACEP COVID-19 Field Guide IHME: COVID-19 Projections ACEP: Now is Not the Time to Reduce Support for Health Care Heroes April 7 CDC Warns of Counterfeit Respirators/ Misrepresentation of NIOSH-Approval September 29 |
COVID-19 Case Dashboard
| Click Here for Current Texas Numbers As of 1/11/2021 at 3:40pm: Cases Reported: 1,730,312 Fatalities: 29,933 Total Tests: 17,148,226 Counties Reporting Cases: 253 of 254 Harris 262,525 -- Dallas 192,567 -- Tarrant 153,472 -- El Paso 103,787 -- Bexar 110,501 -- Travis 55,870 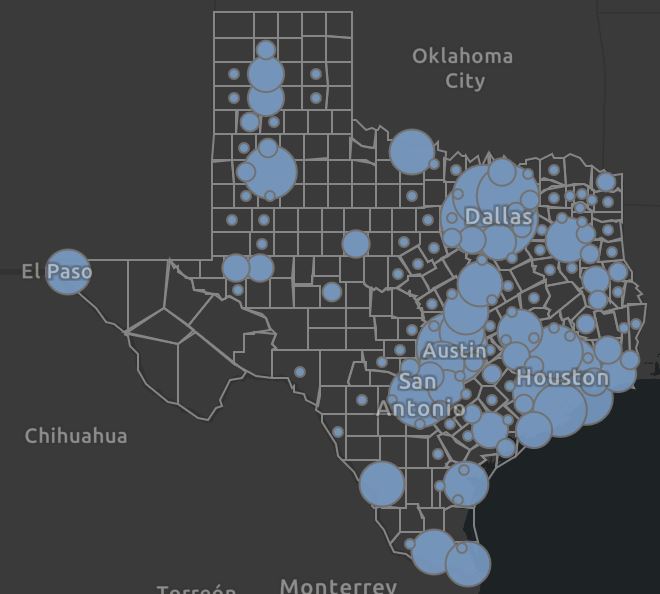 |
View Current Global Numbers As of 1/12/2021 at 5:22am: Total Cases Reported: 90,988,272 US Cases: 22,619,053 Fatalities: 1,947,499 U.S. 376,283 Total Recovered: 50,392,935 Countries/Regions: 191 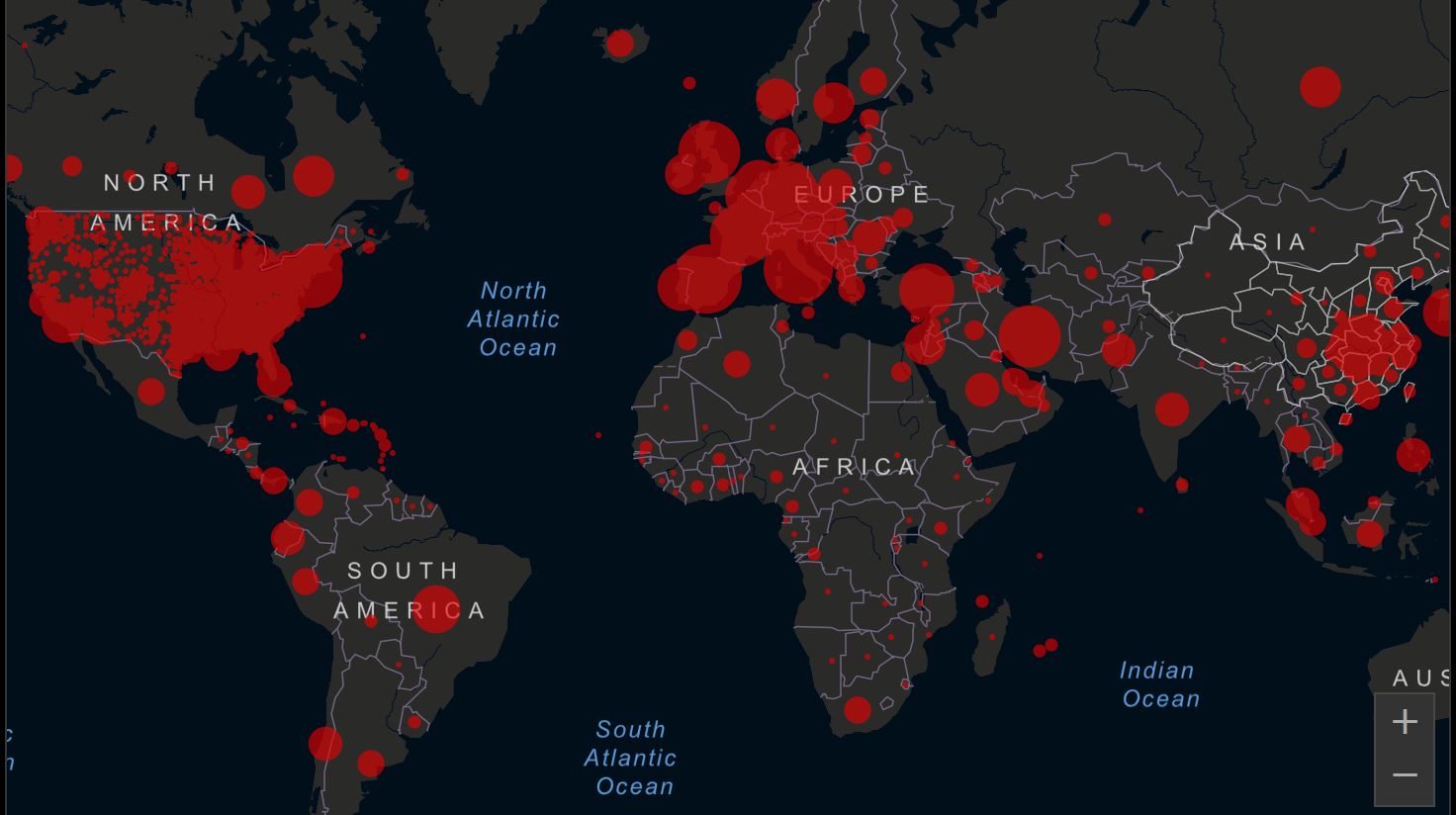 |
COVID-19 Texas Hospital Data
Click Here for Current Hospital Data/Breakdown by Region
As of 1/11/21 at 3:45pm:
Covid Patients in TX Hospitals: 13,397
Total Staffed Hospital Beds: 58,934
Available Hospital Beds: 8,687
Available ICU Beds: 614
Available Ventilators: 6,455
What If My Patient Refuses to Wear a Mask? May 13, 2020
Notice and Compliance Requirements Concerning COVID-19 Minimum Standards of Safe Practice
SNS Requests Go Through RACs
Here is how to contact your Regional Advisory Council to make requests of the Strategic National Stockpile. As a reminder DSHS announced the state has received a share of personal protective equipment from the Strategic National Stockpile. Note that the supplies are being distributed to areas where they are needed most. We are told the supplies are still very limited, for extreme circumstances and will be distributed through certain RACs.
Telehealth Expansion for Medicare
CMS today announced expanded Medicare telehealth coverage that will enable beneficiaries to receive a wider range of healthcare services from their doctors without having to travel to a healthcare facility. Beginning on March 6, 2020, CMS will temporarily pay clinicians to provide telehealth services for beneficiaries residing across the entire country.
Telemedicine and Insurance
Here is Gov. Abbott’s announcement waiving certain regulations and directing that the Texas Department of Insurance issue an emergency rule, all relating to telemedicine care for patients with state-regulated insurance plans to help doctors across Texas continue to treat their patients while mitigating the spread of COVID-19. The suspensions and emergency rule will work together to allow telemedicine visits for patients with state-regulated plans to be paid the same as in-office visits for insurance purposes.
- TDI emergency rule (3/18/2020)
- FAQs on TDI rule (3/18/2020)
Telemedicine and Controlled Substances
The Drug Enforcement Administration has confirmed that telemedicine can now be used under the conditions outlined in the Controlled Substances Act under the public health emergency telemedicine exception to the Ryan Haight Act. DEA-registered prescribers may issue prescriptions for controlled substances via telemedicine without a prior in-person evaluation if the prescription is for a legitimate medical purpose, real-time two-way audio-video is used, and the practitioner is acting in accordance with state law. See more information on this coronavirus page under "telemedicine.”
CDC Discontinuation of Isolation for Persons with COVID-19 Not in Healthcare Settings
Updates as of July 20, 2020:
A test-based strategy is no longer recommended to determine when to discontinue home isolation, except in certain circumstances.
Time-since-illness-onset and time-since-recovery strategy (non-test-based strategy)
See Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for 2019 Novel Coronavirus (2019-nCoV)for specimen collection guidance.
For patients with severe illness, duration of isolation for up to 20 days after symptom onset may be warrented. Consider consultation with infection control experts.
Individuals with laboratory-confirmed COVID-19 who have not had any symptoms may discontinue home isolation when at least 10 days have passed since the date of their first positive RT-PCR test for SARS-CoV-2 RNA, and have had no subsequent illness.j
A summary of current evidence and rationale for these changes is described in the Duration of Isolation and Precautions for Adults with COVID-19.
www.cdc.gov/coronavirus/2019-ncov/hcp/...
ACEP's redesigned COVID-19 clinical alert page
What You Need to Know:
PPE Resources – Respirators
- OSHA Temporary Enforcement Guidance - Healthcare Respiratory Protection Annual Fit-Testing for N95 During the COVID-19 Outbreak
- CDC: Strategies for Optimizing Supply of N95 Respirators
- WHO Advice for the Public: When and How to Use Masks
PPE Resources – Don & Doff
- How to Don and Doff PPE (Fight COVID-19 Coronavirus)
- Donning and Doffing PPE: HCID Level One Full Barrier Isolation
- Demonstration of donning and removing of the appropriate PPE when caring for a person who has COVID-19.
What You Can Do:
- Ensure that your hospital has contacted your state health departments or emergency management agencies to know of your PPE needs. The Strategic National Stockpile is now distributing PPE to the states.
- Send your members of Congress an email urging them to ensure PPE is prioritized for frontline personnel.
- Share your state and federal advocacy efforts on social media with the hashtag #FreeThePPE and encourage others to also contact their representatives.
What ACEP Is Doing:
- Sent letters to Congress and other policymakers with key policy changes necessary to mitigate the impact and spread of the virus in the U.S. and support emergency physicians.
- Hosted a virtual briefing for Congressional health staffers with several WA ACEP members to help inform Congressional efforts on the issue.
- Sent a letter to U.S. Health & Human Services Secretary Alex Azar, outlining specific changes and regulatory waivers the Administration could immediately take to better protect emergency physicians, as well as areas where we want to see additional guidance - including liability immunity.
- Working with CMS and others to ensure that telehealth provisions included in the recently passed Appropriations package in Congress will enable emergency physician use of this valuable tool during the COVID-19 pandemic.
- Attending an upcoming White House meeting with Vice President Pence this week.
Get Real-Time Support:
- The risks you take each day can take a toll on you – a reality heightened during this pandemic. ACEP members receive 3 free counseling sessions when it’s time to practice self-care.
Join more than 2,200 ACEP members sharing strategies and getting peer support on our COVID-19 engagED community forum.
In response to public health and safety concerns about the appropriateness of decontaminating certain respirators, the FDA is reissuing certain EUAs to specify which respirators are appropriate for decontamination. Based on the FDA's increased understanding of the performance and design of these respirators, the FDA has decided that certain respirators should not be decontaminated for reuse by health care personnel. For example, the FDA has learned from the CDC's NIOSH testing that authorized respirators manufactured in China may vary in their design and performance. As such, the FDA has determined that the available information does not support the decontamination of these respirators and has accordingly revised the relevant EUAs. In addition, the FDA is also revising relevant EUAs to no longer authorize decontamination or reuse of respirators that have exhalation valves.